
Health and Medical Sciences
Researchers from the School of Biomedical Sciences at the LKS Faculty of Medicine, the University of Hong Kong (HKUMed), have made a significant advancement that could reshape the treatment landscape for hepatocellular carcinoma (HCC), the most common type of liver cancer, which often resists treatment and recurs. This cancer is especially prevalent in Southeast Asia and China. The research, published in the journal Science Translational Medicine^, revealed a previously unknown mechanism that allows HCC to grow more aggressively and evade existing therapies. The team also developed a new small molecule inhibitor that could improve treatment options for HCC patients.
Unravelling the mystery of tumour resistance
The liver plays a crucial role in various metabolic functions. In HCC, cancerous cells significantly alter these metabolic processes fuelling tumour growth. ‘In our study, we found that a specific metabolic pathway helps cancer cells maintain their stemness, which refer to their enhanced ability to grow, spread and survive,’ explained Professor Stephanie Ma Kwai-yee, co-lead author of the study, and Jimmy and Emily Tang Professor in Molecular Genetics in the School of Biomedical Sciences at HKUMed. Professor Ma is also Associate Vice-President (Research and Innovation) at HKU.
This pathway involves a protein called AGPAT4, which is abundant in both embryonic stem cells and HCC tumour cells, but is rarely found in normal tissues. ‘Our data show that AGPAT4 acts like a switch that makes cancer cells more flexible and aggressive,’ said Professor Ma. ‘This flexibility, known as tumour plasticity, is linked to a higher chance of the cancer to recur, spread and resist existing treatments like sorafenib, a widely used drug for liver cancer.’
The research team discovered that AGPAT4 increases the conversion of a fat molecule called LPA (lysophosphatidic acid) into another molecule called PA (phosphatidic acid). This process activates a signalling pathway involving cell manager mTOR and other proteins, which promote tumour growth and survival. Professor Ma added, ‘When we blocked AGPAT4 in a mouse model, tumour growth slowed down and the cancer became more sensitive to sorafenib.’
Developing an inhibitor to target AGPAT4
Building on these findings, the team developed a compound called CL26, which specifically targets AGPAT4. In preclinical models using patient-derived tumours, CL26 worked in combination with sorafenib to significantly suppress tumour growth. Toxicological assessments showed minimal side effects, suggesting the compound may be safe for future clinical use.
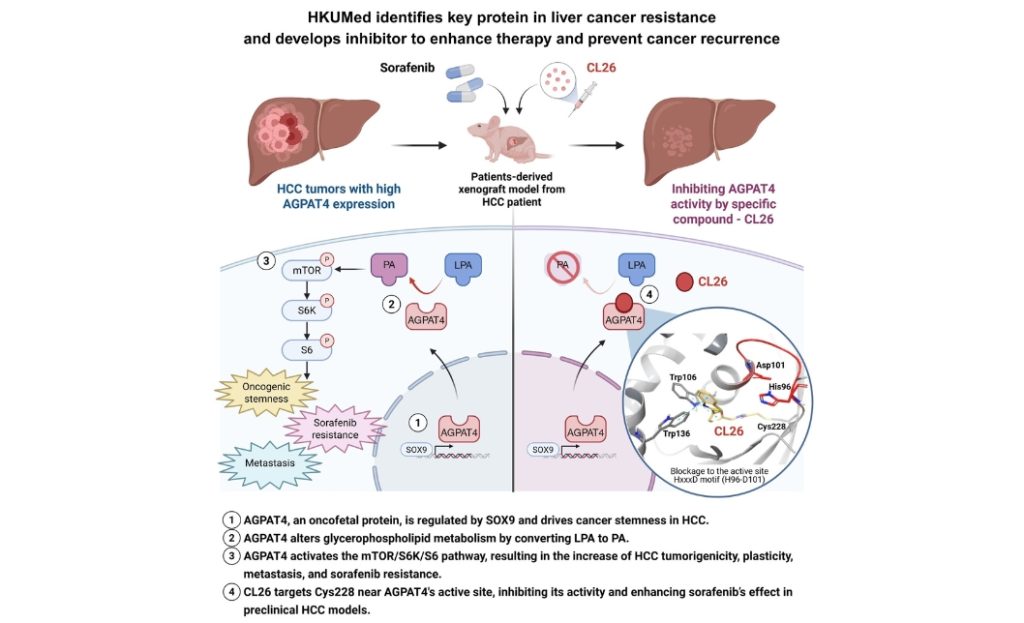
‘This is a promising step forward,’ said Professor Clive Chung Yik-sham, Assistant Professor in the School of Biomedical Sciences, HKUMed, who also co-led the study. ‘CL26 allows us to target liver cancer cells with precision, potentially improving outcomes for patients who no longer respond to standard treatments.’
Eyes on clinical trials to validate treatment potential
‘Our research suggests that AGPAT4 helps HCC cancer cells become more adaptable and resistant to therapy,’ said Professor Ma. ‘By targeting this protein, we may enhance the effectiveness of sorafenib and expand the therapeutic options for patients in clinical settings. We aim to offer hope to countless patients battling this challenging disease.’
Professor Chung added, ‘CL26’s ability to precisely target AGPAT4 is likely one of the key reasons it works so well. We are conducting larger-scale preclinical studies to further assess the efficacy and safety of CL26 and hope to advance to investigational new drug studies and clinical trials.’
About the research team
The collaborative study was co-led by Professor Stephanie Ma Kwai-yee and Professor Clive Chung Yik-sham from the School of Biomedical Sciences at HKUMed. The co-first authors were Dr Johnson Ng Kai-yu and Amy Koo Tin-yan. Major collaborators included professors from The Hong Kong Polytechnic University, the Fourth Military Medical University in Xi’an, the Sun Yat-sen University Cancer Centre, and Guangzhou Medical University in Guangzhou; and Professor Guan Xinyuan from the Department of Clinical Oncology of the School of Clinical Medicine at HKUMed.

Acknowledgements
This research was funded by various grants from the Hong Kong Research Grants Council, including the Theme-Based Research Scheme, the Research Impact Fund, the Collaborative Research Fund, the General Research Fund, the Early Career Scheme and the Research Fellow Scheme. Additional support was provided by the Croucher Foundation’s Senior Research Fellowship and the Guangdong Science and Technology Department. The project also received separate grants through the Innovation and Technology Support Programme under the Innovation and Technology Fund, as well as funding for the Laboratory for Synthetic Chemistry and Chemical Biology, the Centre for Oncology and Immunology, and the Centre for Translational and Stem Cell Biology under the Health@InnoHK Programme initiated by the Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region of the People’s Republic of China.
^Link to the Science Translational Medicine research: https://www.science.org/doi/10.1126/scitranslmed.adn9472
Other Research & Achievements Stories

Achievements and Milestones
Professor Ferenc Krausz, Nobel Laureate in Physics, joined HKU in November 2025 as Professor, Chair of Laser Physics in the Department of Physics under the Faculty of Science. As a world-renowned authority in ultrafast laser science and quantum optics, Professor Krausz’s appointment will strengthen HKU’s capabilities in cutting-edge research and advance innovations in medical diagnostics worldwide. His joining aligns with HKU’s vision of pursuing academic excellence and advancing knowledge, and it is anticipated that he will make significant contributions to scientific development, benefitting both Hong Kong and the global community. In an exclusive interview, Professor Krausz shared his inspiring journey and insights into the future of scientific discovery.
Natural Science
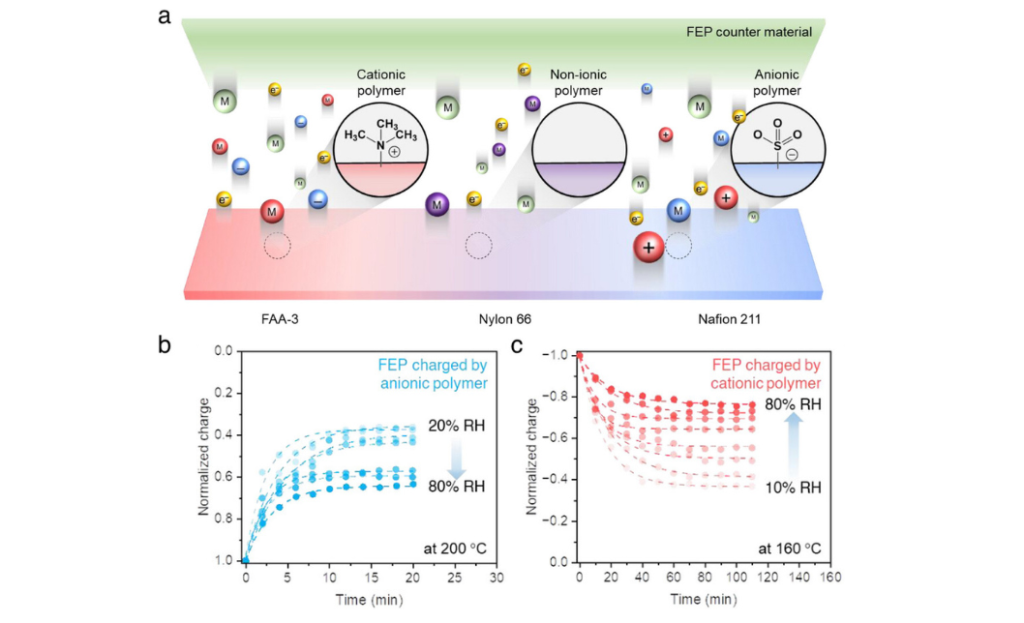
An engineering team at HKU has pioneered the discovery of a new mechanism of ion-driven charge transfer, which provides a deeper understanding of contact electrification phenomena and reveals that electrons and ions transfer simultaneously under changing humidity conditions. This insight will aid in developing new strategies to create materials that work reliably even in humid environments. The findings not only advance fundamental scientific understanding but also open up new possibilities for eco-friendly technologies such as printing, coating, environmental monitoring, and energy harvesting.

Health and Medical Sciences
A research team from HKUMed has pioneered a model using artificial intelligence (AI) to identify sperm with fertilisation potential, achieving a clinical validation accuracy rate exceeding 96%. This AI model automatically selects high-quality sperm based on the morphological features of sperm binding to the egg's zona pellucida (ZP), outperforming traditional methods in terms of speed and reliability, reducing human error, and significantly enhancing the precision of male fertility assessment — ultimately increasing the success rates of assisted reproductive procedures.

Environment and Sustainability
A Hakka village in Guangxi gets abundant rainfall but also struggles with a critical shortage of fresh water and hygiene problems. HKU’s Project Mingde addressed these challenges with the award-winning Duling Educational and Cultural Centre, which harnesses rainfall through innovative water harvesting systems, including tiered roofs, a lotus pond, and underground recycling. The initiative exemplifies interdisciplinary collaboration, cultural sensitivity, and a commitment to social impact, earning international recognition such as the Architizer A+ Jury Award and the Architecture MasterPrize. Overall, it showcases how thoughtful architecture and engineering can turn environmental challenges into sustainable opportunities, fostering resilience and cultural preservation.